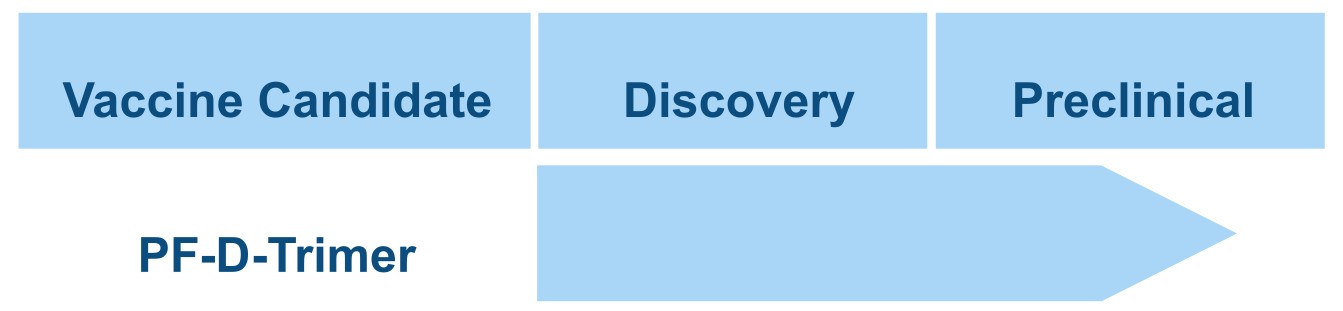
Discovery and Development
Home | Discovery and Development
Human Vaccines
TD™ Trimer-Domain Technology Platform
TD™ Trimer-Domain is a proprietary protein domain that both stabilizes the trimeric structure of the protein and also allows its purification in one easy step.
Key features of TD™ Trimer-Domain:
- Development of protein-based vaccines according to the natural trimerized structure.
- High-expression in standard CHO cells based bioprocesses.
- Rapid one-step purification process.
- Currently in an Investigator-Initiated Trial (IIT).
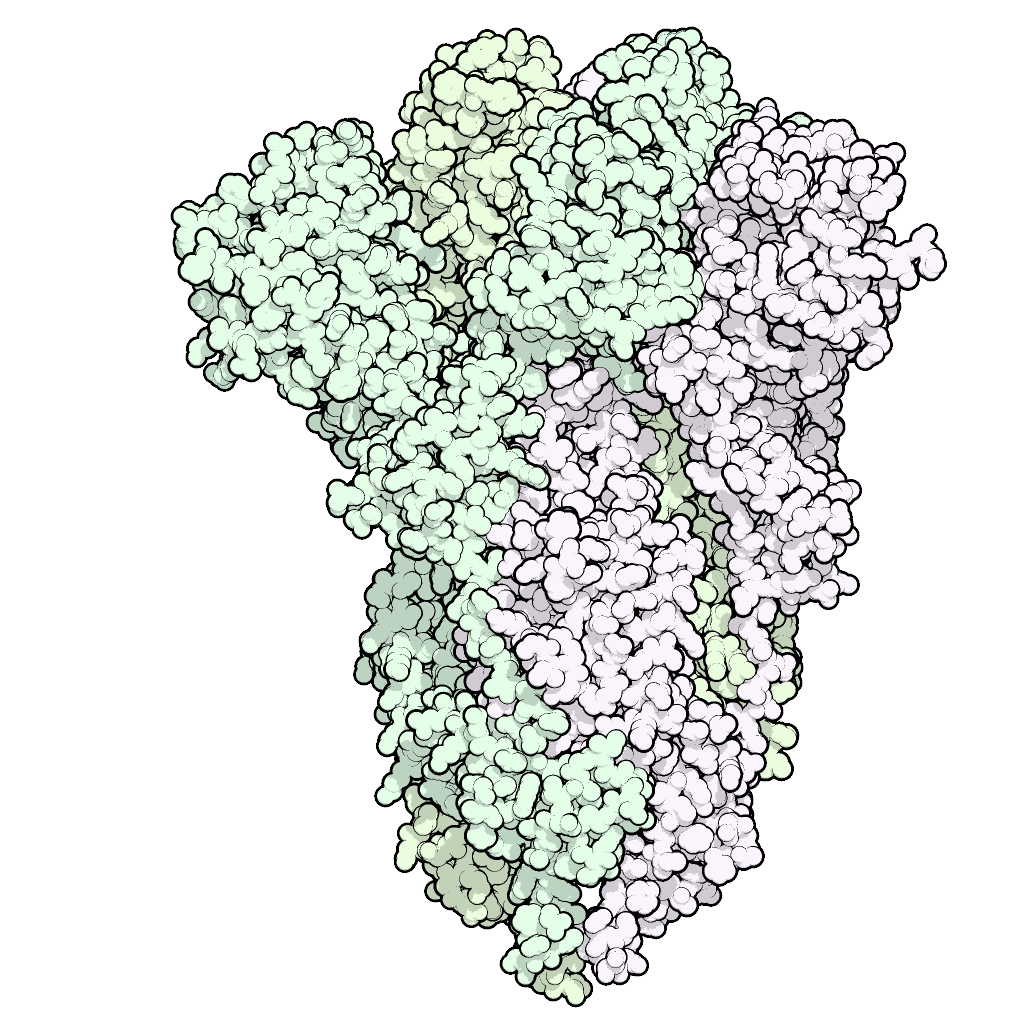
Our human vaccine candidate, SARS-CoV-2 PF-D-Trimer™, consists of the TD™ Trimer-Domain stabilized recombinant viral S-glycoprotein in its prefusion trimeric form. Major proteins of many viruses responsible for human and veterinary diseases, including the S protein of coronaviruses, only exist in a trimeric form.
We are also developing a trimeric vaccine based on the hemagglutinin of the influenza virus. This vaccine candidate also uses the TD™ Trimer-Domain technology.
Veterinary Vaccines
Virus-Like Particles and Subunit Vaccines
Veterinary vaccines have played and continue to play a major role in protecting animal and public health and enable efficient production of food for the burgeoning human population. Animal vaccines can be developed and licensed much more quickly than human vaccines.
In order to meet the urgent needs for new veterinary vaccines, Nova Biologiques has developed high-efficiency, low-cost VLP and subunit therapeutic solutions.
One of these vaccines, currently in preclinical studies, protects against Classical swine fever (CSF), one of the most devastating viral infectious diseases affecting members of the Suidae family, which has serious repercussions on the world economy.
We have also developed VLP vaccines for protection against avian influenza and Newcastle disease viruses, two important pathogens in poultry worldwide.
Human Clinical Trials
Prophylactic SARS-CoV-2 Vaccine Candidate: PF-D-Trimer™, a broad protective SARS-CoV-2 subunit vaccine developed from our TD™ Trimer Domain platform using the Delta spike protein.
- Investigator-Initiated Trial (IIT) in collaboration with our Chinese partners, to evaluate the safety, tolerability, and immunogenicity of PF-D-Trimer™.
- The data continues to strengthen the potential of the TD™ platform against coronaviruses and against other viral diseases.
- The results from the studies are expected by the end of 2022.